Continuing our series on the social and scientific basis of metabolic rift theory.
On every scale, from the smallest cells to the entire planet, the essential elements of life are constantly used and re-used. Biogeochemical cycles are the basis of the biosphere.
by Ian Angus
If it weren’t for recycling, you would be dead.
Like every other animal, you depend on the tiny energy factories found in every cell — the mitochondria — to produce the energy you need. Similarly, every green plant depends on chloroplasts to convert sunlight into the chemical energy it needs.
Animals and plants both store their energy in Adenosine Triphosphate (ATP), a complex molecule that is often called life’s energy currency. Triphosphate means it contains three phosphate molecules. It takes energy to hold ATP together, energy that can be released by splitting off one of the phosphate molecules. That’s what the cells in your body do whenever they need to do anything. The ATP is thus “spent” — with only two phosphate molecules, it becomes ADP, Adenosine Diphosphate.
Life needs a lot energy: every cell in your body consumes about 10 million ATP molecules a second. In total, an adult human body uses its full weight (60-75 kilograms) in ATP every day. Since there are only about 250 grams (8 ounces) present at any given time, mitochondria must recycle every molecule of ADP repeatedly, adding phosphate to make ATP again. Every molecule is recycled 500 to 750 times a day.
ATP and ADP are at the core of metabolism. Without the energy they provide, you could not move or digest or breathe or see or think. If the ATP recycling process stopped, you would not last long, nor would any other living thing.
The ATP/ADP energy cycle was discovered in the twentieth century, but it is an ancient process, invented by bacteria over three billion years ago. It is a prime example of how, from life’s beginnings, metabolism has endlessly recycled materials: some processes are themselves circular, others are paired with opposite processes that return material to the starting point. ATP is particularly important, but its production and recycling is only one of hundreds of metabolic processes going on constantly, at various speeds, in every cell.
Figure 1 is a conceptual map of some cellular metabolic pathways. As it shows, the processes are not only complex, they are interlinked, with each cycle connected to and dependent upon others.
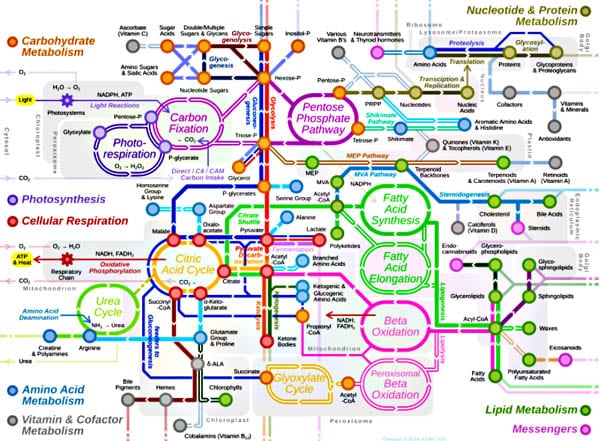
Figure 1. A simplified map of some cellular metabolic cycles. For interactive maps that are more detailed but still far from comprehensive, see http://biochemical-pathways.com/#/map/1.
These essential life cycles take place at the smallest possible scale — molecules interacting inside cells — and at spectacular speeds.
Earth’s life-support systems also involve planet-wide cycles that operate over time spans ranging from minutes to millions of years. As we will see in future articles, the micro and macro levels are tightly interlocked.
‘A metabolism prescribed by the natural laws of life’
The idea that life depends on recycling isn’t new. Over 2000 years ago, the Roman philosopher/poet Lucretius described recycling as a fundamental principle of the universe:
“Things, therefore, do not utterly perish, which seem to do so, since Nature recruits one thing from another, nor suffers any thing to be produced, unless its production be furthered by the death of another… I have shown that things cannot be produced from nothing and also that when produced they cannot return to nothing.”[1]
In 1260 CE, the Arab physician Ibn al-Nafis offered the earliest definition of metabolism:
“the body and all its parts are in a continuous state of dissolution and nourishment, so they are inevitably undergoing permanent change.”[2]
Despite such early intuitions, scientific understanding of life’s metabolism was minimal until the nineteenth century. The difference between living and non-living matter was usually explained by vitalism, the essentially religious view that all living things contain an undetectable vital fluid or life-force. Vitalists held that the complex carbon compounds found in plants and animals could only be created if the mysterious vital fluid was present — the term “organic chemistry” for the study of carbon compounds originated in that mistaken view.
The modern life sciences originated in a series of major discoveries in the late 1700s and early 1800s. In the 1770s, after Joseph Priestly showed that fire removes something essential for life from the air and green plants restore it, Antoine Lavoisier isolated the “something” and named it oxygen. In 1789, Jan Ingenhousz showed that plants use energy from light to absorb carbon dioxide and release oxygen. In 1828, Friedrich Wöhler did what vitalist theory said was impossible, by synthesizing an organic compound (urea) in his laboratory. In the 1830s, Matthias Schleiden and Theodor Schwann showed that all plants and animals are composed of living cells — that “there is one universal principle of development for the elementary parts of organisms, however different, and that this principle is the formation of cells.”[3]
In the 1840s, Julius Robert Mayer discovered the law of conservation of energy and showed, in The Relation of Organic Motion to Metabolism, that it applied to living things: photosynthesis doesn’t create new energy, but rather converts light energy into chemical energy.
These and many other discoveries contributed to a science of metabolism, a concept so new that English didn’t even have a word for it until the 1870s.[4]
A leading figure in much of this research was the German chemist Justus Liebig.[5] Now known as the father of organic chemistry, he personally published 300 papers on the subject between 1830 and 1840, and over 2,000 in his lifetime. As his student Carl Voit later wrote:
“Liebig was the first to establish the importance of chemical transformations in the body. He stated that the phenomena of motion and activity which we call life arise from the interaction of oxygen, food and the components of the body. He clearly saw the relation between metabolism and activity and that not only heat but all movement was derived from metabolism. He investigated the chemical processes of life and followed them step by step to their excretion products.”[6]
From 1840 on, Liebig focused his attention on agriculture, applying chemical analysis to understand and help overcome declining soil fertility across Europe. He showed that, contrary to widespread opinion, plants were not nourished by absorbing decayed organic matter (humus) through their roots. Rather, roots took up water that contained specific non-organic elements, and combined those with carbon and oxygen from the air to build plant matter.
Traditionally, food grown on farms was consumed locally, so its elements were returned to the land as food waste and excrement, nourishing future crops. The shift to market-oriented farming changed that: most crops were produced for sale and consumed in distant cities, where food waste and excrement were discarded. In Marx’s words, “in London … they can do nothing better with the excrement produced by 4½ million people than pollute the Thames with it, at monstrous expense.”[7]
Result: essential nutrients in food were not returned to the soil, leading to a long-term decline in fertility. In Liebig’s view, artificial fertilizers were a best a temporary solution: agriculture would continue to decline unless ways were found to use excrement instead of discarding it.
As John Bellamy Foster and Kohei Saito have shown, Karl Marx studied the work of Liebig and other agricultural scientists carefully, and incorporated the concepts of metabolism and metabolic cycles into his analysis of capitalism.[8] In his view, “to have developed from the point of view of natural science the negative, i.e., destructive side of agriculture, is one of Liebig’s immortal merits.”[9]
From Liebig, Marx and Engels learned how capitalist agriculture “produces conditions that provoke an irreparable rift in the interdependent process of social metabolism, a metabolism prescribed by the natural laws of life itself.”[10]
Marx’s “metabolism prescribed by the natural laws of life itself” was precisely Liebig’s view that “rational agriculture … is based upon the principle of restitution; by giving back to the fields the conditions of their fertility, the farmer insures the permanence of the latter.”[11] Rather than rational agriculture, Liebig wrote, European (especially English) farming was a robbery system, in which essential elements were stolen from the soil and never returned, impoverishing the land. The only long-term solution would be to recreate, in the new social conditions, the natural cycles that had made agriculture possible in the first place — to heal the metabolic rift between human society and a critically important life-support system.
“If it were practicable to collect, without the least loss, all the solid and fluid excrements of the inhabitants of towns; and to return to each farmer the portion arising from produce originally supplied by him to the town, the productiveness of his land might be maintained almost unimpaired for ages to come, and the existing store of mineral elements in every fertile field would be amply sufficient for the wants of the increasing populations.”[12]
Liebig’s view of soil fertility was part of a general conception that life depended on recycling. “All the innumerable products of vitality resume, after death, the original form from which they sprung. And thus death — the complete dissolution of an existing generation — becomes the sources of life for a new one.”[13]
Vaclav Smil says that such statements show that Justus von Liebig was “one of the first scientists to offer a coherent image of global biospheric cycles.”[14]
Liebig’s blind spot
Smil is correct that Liebig’s work and ideas were a major step towards understanding the cyclical processes that comprise Earth’s life-support systems. However, that understanding couldn’t develop fully until other scientists got past a major misconception that prevented Liebig from understanding how the cycles work.
Chemical analysis showed that all plants contain nitrogen, leading Liebig to conclude, correctly, that nitrogen is one of a handful of elements that are essential for plant growth. Plants simply cannot live without it. The question was, where did it come from? Liebig found no source in soil, and only small amounts in animal manure, so he concluded, reasonably enough, that it must come from the air. Air is less than one percent carbon dioxide and more than 78 percent nitrogen: if plants can get all of the carbon dioxide they need from air, why not nitrogen as well?
Atmospheric nitrogen is inert — under normal conditions, it won’t combine with any other chemical — but lightning can force it to combine with oxygen, producing nitrogen oxides, which are soluble in water. Liebig argued that plants obtain nitrogen that was created by lightning and dissolved in rain. As a result, he believed, there was no need to include nitrogen in fertilizers, because plants could get all they needed from the air.
That was a reasonable assumption, but it was wrong. Chemical analysis showed that there was far too little nitrogen in rain to nourish plants, and practical experiments showed that fertilizers containing nitrogen promoted far more plant growth than those without it. Liebig was right that plants need nitrogen, but wrong (although he refused to admit it) about where they found it. (His critics, we should note, had no better idea than he about where unfertilized plants obtained nitrogen.)
The answer was not found until after Liebig’s death, but he might have found it earlier, or at least contributed to the solution, if he had not been committed to purely chemical explanations of metabolism. In the 1840s, he attributed both the decomposition of dead things and human diseases to “fermentation” which he insisted was a purely chemical process. He ridiculed the suggestion that yeast is alive. In the 1850s, he refused to accept Louis Pasteur’s demonstration that microorganisms were responsible for spoiling wine and beer. One of his last major projects, in 1868, was a series of lectures criticizing Pasteur’s work and denying that bacteria played any role in fermentation, decay or disease. As Frederick Engels noted, these and other arguments showed “how much of a dilettante Liebig was in biology, although the latter is a science bordering on chemistry.”[15]
The science Pasteur founded — microbiology — solved the nitrogen puzzle. By 1888, scientists had proven conclusively that the nitrogen used by plants did come from the air, but not directly, as Liebig thought. First, certain species of bacteria, most of which live in the roots of legumes such as beans and clover, capture and “fix” nitrogen into compounds that plants can use.[16] The amount of nitrogen available is limited because only a few types of bacteria can do this, and because over time, other bacteria convert the nitrogen back to its original inert form, and return it to the atmosphere.[17]
The discovery of this nitrogen cycle, together with the realization that bacteria are also responsible for the eventual decomposition of all organisms, showed that life is a major factor — an essential driver — in the vast recycling operations that make life possible. Earth’s metabolism isn’t just biological or geological or chemical — it is all of those at once.
In 1922, the Russian geochemist Vladimir Vernadsky gave these ubiquitous processes a compound name: biogeochemistry. As he later wrote, “there is a continual migration of atoms from inert matter to living matter and back again … Biogeochemical phenomena are the basis of the biosphere.”[18]
Future articles in this series will examine the global biogeochemical cycles that comprise Earth’s life-support systems, and discuss how capitalism is disrupting them, with potentially disastrous consequences.
Notes
[1] Lucretius, On the Nature of Things, prose translation of De Rerum Natura by John Selby Watson, (London: Henry Bohn, 1851), 15.
[2] Quoted in David Gerow Irving, The Protein Myth (Washington: John Hunt, 2011), 188.
[3] Theodore Schwann, quoted in Stephen F. Mason, A History of the Sciences (New York: Collier, 1962), 389.
[4] The German word Stoffwechsel became widely used in the 1840s. See Marx and Metabolism: Lost in translation?
[5] He became Justus von Liebig when he was awarded the title Freiherr (Baron) by Ludwig II of Bavaria in 1845.
[6] Quoted in George Rosen, “The conservation of energy and the study of metabolism,” in Brooks Chandler and Paul Cranefield, eds., The historical development of physiological thought (New York: Hafner’ 1959), 258.
[7] Karl Marx, Capital Volume 3 (London: Penguin, 1981 [1894]), 195.
[8] John Bellamy Foster, Marx’s Ecology (New York: Monthly Review Press’ 2000); Kohei Saito, Karl Marx’s Ecosocialism (New York: Monthly Review Press, 2017)
[9] Karl Marx, Capital Volume 1 (London: Penguin, 1976 [1867]) 638.
[10] Karl Marx, Capital Volume 3 (London: Penguin, 1981 [1894]), 949
[11] Justus von Liebig, Letters on Modern Agriculture (New York: John Wiley, 1959) 144
[12] Justus von Liebig, The Natural Laws of Husbandry (London: Walton & Maberly,1863), 274
[13] Justus Liebig, Organic Chemistry in its Applications to Agriculture and Physiology (London: Taylor and Walton, 1840) 91-2. Carbonic acid is carbon dioxide. Ammonia is a source of nitrogen.
[14] Vaclav Smil, Enriching the Earth (Cambridge: MIT Press, 2001), 8.
[15] Frederick Engels, Dialectics of Nature (New York: International Publishers, 1940) 192-3.
[16] 20th century research showed that some ocean-dwelling bacteria fix nitrogen as well.
[17] The nitrogen cycle is actually much more complex than this. More in future articles.
[18] Quoted in Vaclav Smil, Cycles of Life (New York: Scientific American Library, 1997), 6.



Ian’s article shows the importance of understanding natural cycles, especially nutrient cycles, if we are to overcome the dislocations, pollution and soil death promoted by capitalist agriculture. I have a few comments.
Firstly, it is stated that “it was known that lightning forced it [nitrogen] to combine with hydrogen, producing ammonia (NH3)”. This may be have thought at the time, but I can find no modern study that suggests this really happens. Modern work seems to indicate that atmospheric ammonia (pollution) derives almost exclusively from agriculture – fertilisers and manure. It would be thermodynamically very unfavourable to produce nitrogen from lightning: there is almost no free hydrogen in the atmosphere, as the molecules are too light to to be held there by gravity and the presence of oxygen provides a competing reaction, which is known to make millions of tonnes of NOx annually.
Secondly, it is stated here that bacteria are responsible for the eventual decomposition of all organisms, but I would have thought that fungi have a role.
Finally, to back up Ian’s point about Liebig’s blind spot, if you look on Google Books for William H Brock: Justus Liebig, the Chemical Gatekeeper, pages 173-4, you will find a remarkable table showing the manure is just as effective in promoting wheat yields as artificial fertilisers, from a Rothamstead study between 1844-1856. Of course, this agricultural station developed a commercial interest in promoting the latter.
Phil …
You are right. What I should have said was that lightning can break dinitrogen molecules and combine them with oxygen to create nitrogen oxides, which later contribute to ammonia formation in the soil. I have corrected that paragraph.
The issue you refer to — ammonia pollution arising from agriculture — is an entirely separate matter, a result of overuse of artificial fertilizers in the past century: it was not a factor in Liebig’s time.
Yes enzymes from fungi play an important role in decomposition, but that was not known in the 1800s, so it wasn’t a factor in the early recognition of the role of life in biospheric cycles, and that’s what I was writing about.
A future article in this series will address nutrient cycles further.
Ian, thank you for this article and the entire series. A question that arises in local food movement discussions about the “metabolic rift” concept has to do with how to build up local soil quality for growing food on marginal land. Many people are doing home composting and adding that plus biochar and sometimes human urine to the soil in their vegetable gardens. There’s lots of debate about whether to not these methods can be scaled-up to replacefood imported from outside the region.
I have a question related to the article above concerning how plants actually obtain nurishment from efforts to retain food waste locally – essentially local efforts to heal that part of the metabolic rift.
The article references Liebig’s contention that … “contrary to widespread opinion, plants were not nourished by absorbing decayed organic matter (humus) through their roots. Rather, roots took up water that contained specific non-organic elements, and combined those with carbon and oxygen from the air to build plant matter.
You then go on to say …”(T)raditionally, food grown on farms was consumed locally, so its elements were returned to the land as food waste and excrement, nourishing future crops.
My question is this. If it’s not the humus that delivers the required nutrients to the roots systems directly but rather the water, am I correct to conclude that it’s the water that absorbs the nutrients from the humus for uptake by the roots?
Broadly speaking, yes, but of course humus varies a lot, so it may or may not have all the nutrients needed. And whether the nutrients present can be used depends a lot on what microbes and fungi are in the soil. There’s lots about this on the web. One starting point: https://greentumble.com/what-role-does-humus-play-in-soil-fertility/